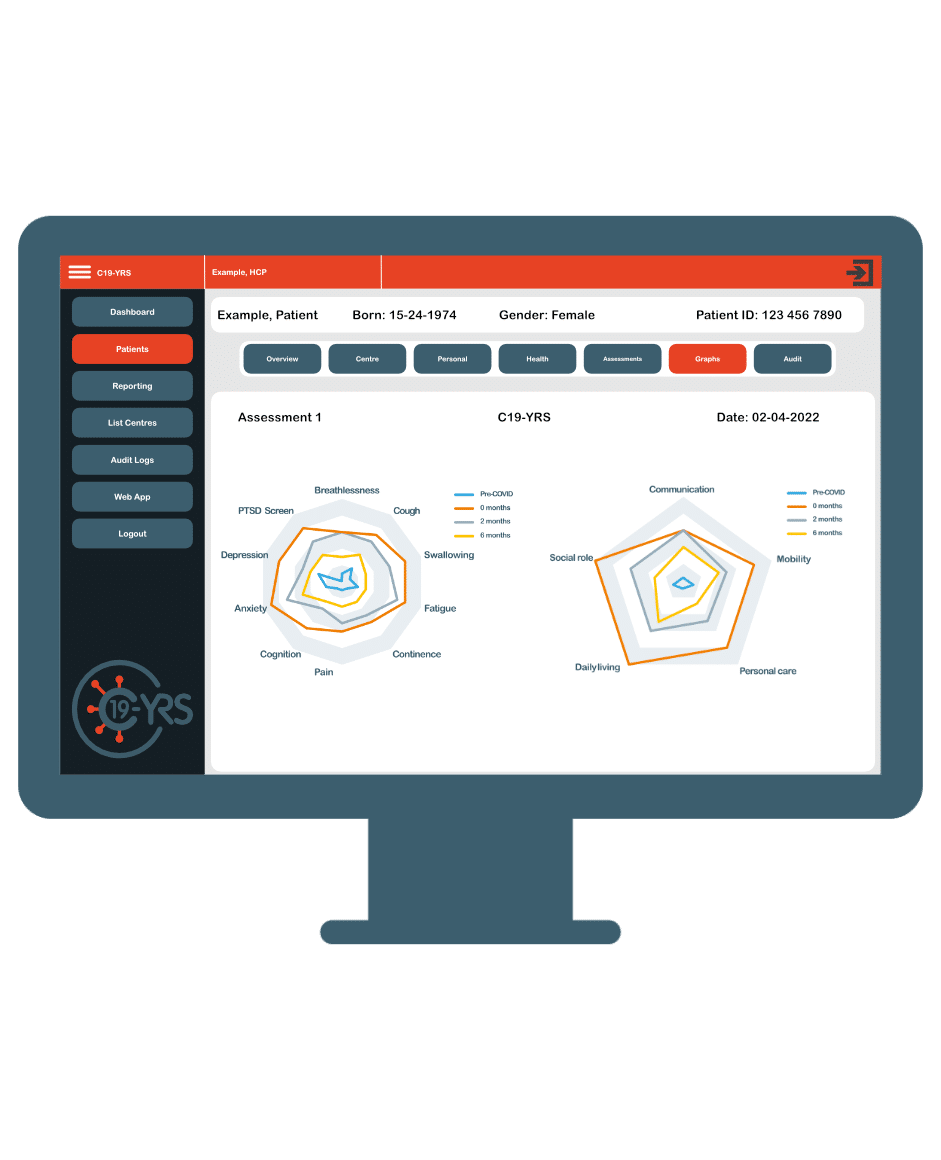
ELAROS C19-YRS
An award-winning digital platform for the remote assessment, triage, monitoring, management and rehabilitation of patients with long term conditions. A not for profit initiative for all public health organisations.
Recommended nationally by NHS England and
NHS Scotland.
Developed with the NHS, available worldwide for clinical, research, or personal use.
Customisable and scalable PROMs, eDiaries, support resources and your local branding to streamline any clinical service, project or personal monitoring.
Book a demo:
c19-yrs@elaros.com